Epicococin H
IUPAC name: dodecahydro-3,4,10,11-tetrahydroxy-6a,13a-bis(methylthio)-, (3S,4R,4aS,6aR,7aR,10S,11R,11aS,13aR,14aR)-1H,6H-Pyrazino[1,2-a:4,5-a']diindole-1,6,8,13(2H,6aH)-tetrone
CAS Number: 1196688-70-6
Class: Cyclodipeptide
Mushrooms contain this compound
1 mushrooms
| MushID |
Thai name |
Common name |
Scientific name |
Family |
| 2 |
ถั่งเช่าทิเบต |
caterpillar fungus |
Cordyceps sinensis (Berk.) Sacc. |
Ophiocordycipitaceae |
2D Structure
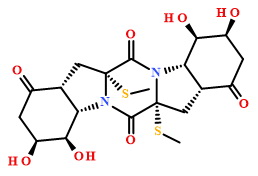
Compound Properties
SMILES
CS[C@@]12C[C@@H]3[C@H](N1C(=O)[C@]1(N(C2=O)[C@H]2[C@@H](C1)C(=O)C[C@@H]([C@@H]2O)O)SC)[C@@H](O)[C@H](CC3=O)O
Physicochemical Properties
| Formula |
C20H26N2O8S2 |
| Molecular wieght(g/mol) |
486.56 |
| Num. of Heavy atoms |
32 |
| Num. of Aromatic Heavy atoms |
0 |
| Fraction Csp3 |
0.80 |
| Num. of Rotatable bonds |
2 |
| Num. of H-bond acceptors |
8 |
| Num. of H-bonds donors |
4 |
| Molar Refractivity |
122.01 |
| TPSA (square Angstrom) |
206.28 |
Lipophilicity
| iLOGP |
0.21 |
| XLOGP3 |
-2.84 |
| WLOGP |
-2.82 |
| MLOGP |
-2.06 |
| Silicos-IT LOGP |
-1.74 |
| Consensus LOGP |
-1.85 |
Water Solubility
| ESOL |
|
| LogS |
-0.94 |
Solubility(mg/ml) |
5.65E+01 |
Solubility(mol/l) |
1.16E-01 |
Class |
Very soluble |
| Ali |
|
| LogS |
-0.94 |
Solubility(mg/ml) |
5.64E+01 |
Solubility(mol/l) |
1.16E-01 |
Class |
Very soluble |
| Silicos-IT |
|
| LogS |
0.24 |
Solubility(mg/ml) |
8.42E+02 |
Solubility(mol/l) |
1.73E+00 |
Class |
Soluble |
Pharmacokinetics
| GI absorbtion |
Low |
| BBB permeant |
No |
| Pgp substrate |
Yes |
| CYP1A2 inhibitor |
No |
| CYP2C19 inhibitor |
No |
| CYP2C9 inhibitor |
No |
| CYP2D6 inhibitor |
No |
| CYP3A4 inhibitor |
No |
| Log Kp (skin permeation) (cm/s) |
-11.28 |
Druglikeness
| Lipinski Num. violations |
0 |
| Ghose Num. violations |
2 |
| Veber Num. violations |
1 |
| Egan Num. violations |
1 |
| Muegge Num. violations |
2 |
| Bioavailability Score |
0.55 |
Medicinal Chemistry
| PAINS Num. of alerts |
0 |
| Brenk Num. of alerts |
0 |
| Leadlikeness Num. of violations |
1 |
| Synthetic Accessibility |
5.50 |
Biological Activity
| Biological Activity |
JID |
| Anti-HIV |
45 |
