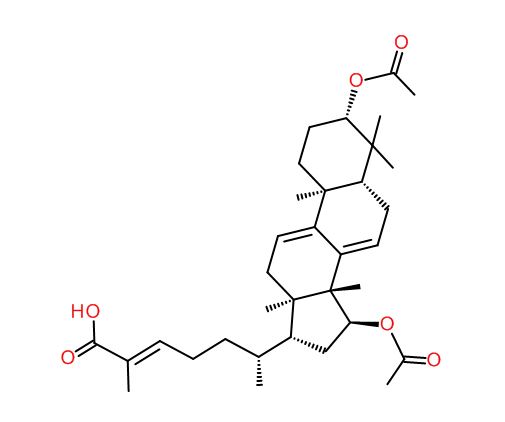
Ganoderic acid S
IUPAC name: (E,6R)-6-[(3S,5R,10S,13R,14R,15S,17R)-3,15-diacetoxy-4,4,10,13,14-pentamethyl-2,3,5,6,12,15,16,17-octahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-methyl-hept-2-enoic acid
CAS Number: 112430-63-4
Class: Terpenoid
Mushrooms contain this compound
1 mushrooms
| MushID |
Thai name |
Common name |
Scientific name |
Family |
| 1 |
เห็ดหลินจือ |
Lingzhi |
Ganoderma lucidum ( Curtis ) P. Karst. |
Ganodermataceae |
2D Structure
Compound Properties
SMILES
CC(=O)O[C@H]1C[C@@H]([C@@]2([C@]1(C)C1=CC[C@@H]3[C@](C1=CC2)(C)CC[C@@H](C3(C)C)OC(=O)C)C)[C@@H](CC/C=C(/C(=O)O)\C)C
Physicochemical Properties
| Formula |
C34H50O6 |
| Molecular wieght(g/mol) |
554.76 |
| Num. of Heavy atoms |
40 |
| Num. of Aromatic Heavy atoms |
0 |
| Fraction Csp3 |
0.74 |
| Num. of Rotatable bonds |
9 |
| Num. of H-bond acceptors |
6 |
| Num. of H-bonds donors |
1 |
| Molar Refractivity |
158.98 |
| TPSA (square Angstrom) |
89.90 |
Lipophilicity
| iLOGP |
3.90 |
| XLOGP3 |
7.12 |
| WLOGP |
7.43 |
| MLOGP |
5.34 |
| Silicos-IT LOGP |
6.54 |
| Consensus LOGP |
6.07 |
Water Solubility
| ESOL |
|
| LogS |
-7.17 |
Solubility(mg/ml) |
3.74E-05 |
Solubility(mol/l) |
6.74E-08 |
Class |
Poorly soluble |
| Ali |
|
| LogS |
-8.83 |
Solubility(mg/ml) |
8.25E-07 |
Solubility(mol/l) |
1.49E-09 |
Class |
Poorly soluble |
| Silicos-IT |
|
| LogS |
-6.07 |
Solubility(mg/ml) |
4.71E-04 |
Solubility(mol/l) |
8.49E-07 |
Class |
Poorly soluble |
Pharmacokinetics
| GI absorbtion |
Low |
| BBB permeant |
No |
| Pgp substrate |
Yes |
| CYP1A2 inhibitor |
No |
| CYP2C19 inhibitor |
No |
| CYP2C9 inhibitor |
Yes |
| CYP2D6 inhibitor |
No |
| CYP3A4 inhibitor |
No |
| Log Kp (skin permeation) (cm/s) |
-4.63 |
Druglikeness
| Lipinski Num. violations |
2 |
| Ghose Num. violations |
4 |
| Veber Num. violations |
0 |
| Egan Num. violations |
1 |
| Muegge Num. violations |
1 |
| Bioavailability Score |
0.56 |
Medicinal Chemistry
| PAINS Num. of alerts |
0 |
| Brenk Num. of alerts |
2 |
| Leadlikeness Num. of violations |
3 |
| Synthetic Accessibility |
6.75 |
Biological Activity
| Biological Activity |
JID |
| Antitumour |
6 |
| Anti-angiogenic |
6 |
| Anti-HIV |
6 |
| Antihistaminic |
6 |
| Anti hepatotoxic |
6 |
| Complement inhibition |
6 |
| Hypocholesterolemic |
6 |
| Antiplatelet aggregation |
6 |
